It is a must-have for any library. Q calor C cal Equation 4 T mix T initial.

Hhyuu Designing A Hand Warmer Lab 4 Page 4 Created With Publitas Com
Designing a hand warmer ap lab answers - Bing OCOOPA Fast-Charging Hand Warmers 10000mAh Handwarmer with PD QC 30 Rechargeable Hand Warmer Supercar Design Heating time 15 Hrs Perfect for Outdoor Activities Brilliant Winter Gift.

. Designing a hand warmer ap lab answers Created Date. To determine the best solute to use to make a safe hand warmer. Use the energy equation to calculate the amount of heat in joules released by the warm water as it cooled.
Thomas Greenbowe Department of Chemistry and Biochemistry. Q hot -18929 J c. 7654 Designing a Hand Warmer AP Chemistry Big Idea 5.
This is a video outlining a Flinn Lab on designing a hand warmer. QmcΔT Published Values for Heat of Solution. 47 out of 5 stars 324.
Designing A Hand Warmer Ap Lab Answers - CalMatters Search this site. Magnesium sulfate would make a better hand warmer because it actually increases the temperature of the water. The calorimeter constant C cal is calculated as follows.
The heat gained by the calorimeter q calor is equal to that lost by the water but opposite in sign. Q hot 99 g 4 JgC -45C b. Table Part B and C.
Of some chemical reactions. Designing A Hand Warmer Ap Lab Answers Keywords. The specific heat of the solution is generally assumed to be the same as that of water namely 418 JgC.
Mass of water specific heat of waterT mix T avg Equation 3 where the mass is the total mass of hot and cool water. Designing a Hand Warmer by Alexis Mabugat. Dictionary com s List of Every Word of the Year.
The only issue with LiCl is that it is very expensive compared to. 7 Calculate the molar heat of solution q solution -q aq q cal -m. Designing a hand warmer ap lab answers - Bing OCOOPA Fast-Charging Hand Warmers 10000mAh Handwarmer with PD QC 30 Rechargeable Hand Warmer Supercar Design Heating time 15 Hrs Perfect for Outdoor Activities Brilliant Winter Gift.
CHEMISTRY Designing a Hand Warmer IN7654 040313 Catalog No. Be mindful of the safety procedures. 47 out of 5 stars 324.
The heat gained by the calorimeter q calor is equal to that lost by the water but opposite in sign. Our hand warmer was an exothermic reaction since it released heat. 1 Measure out 2 separate samples of 1000 mL of distilled water 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds measure temp 4 Repeat DAY 2.
Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is. Thus calorimetry can be used to measure the energy supplied or discarded as heat by a reaction and can identify q with a change in internal energy if the reaction occurs at constant volume or with a change in enthalpy if the. Designing A Hand Warmer Ap Lab Answers Author.
Use chemistry to design an effective safe environmentally benign and inexpensive hand warmer. One type of hand warmer contains a mixture of dry chemicals including among other things powdered iron and salt. Rather than reading a good Page 244.
Using heatresistant gloves measure 1000 mL of the hot water in a 100mL graduated cylinder. Find the training resources you need for all your activities. PDF Chemistry Designing A Hand Warmer Lab Answers AP Chemistry.
The calorimeter constant C cal is calculated as follows. One example is the hand warmer. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch.
Up to 24 cash back Designing a Hand Warmer Lab. Place the calorimeter of stir mixer. Download Ebook Chemistry Designing A Hand Warmer Lab Answers engineers scientists chemists and students this volume is applicable to many different fields across many different industries at all levels.
Create a hand warmer using a substance that is safe cheap and emits the most heat substances are lithium chloride calcium chloride and sodium carbonate pour 5 grams of the substance and 45 mL of water into a cup cover the cup and record the temperature using a thermoprobe solve the equation. The salt dissolves and the water warms. It was created by Alex Brinley Charis Conwell and Siena Joy for our AP Chemistry class.
At this point was immediately and carefully removed using tongs and the mass of the water in the calorimeter was measured154 g. U M School of Public Health News. Car News Reviews amp Pricing for Environmentally Friendly.
Q m s ΔT Equation 1. A pre-lab assignment enabling you to easily incorporate lab safety into all your lab courses without building in additional teaching time. 1 measure 40mL of distilled water into a styrofoam cup on stir plate 2 record temp of water.
Answer the following questions in your lab notebook. Read Book Designing A Hand Warmer Ap Lab Answersthan the PDF Chemistry Designing A Hand Warmer Lab Answers WHS AP Chemistry. Designing A Hand Warmer Ap Lab Answers Spencer J.
The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a. There are several types of hand warmers commercially available that take advantage of different chemical reactions to provide a gentle heat for an extended period of time. Up to 24 cash back mass of water x specific heat of water x T mix T avg Equation 3 where the mass is the total mass of hot and cool water.
AP MANUAL 2013 p. Where m is the total mass of the solution solute plus solvent s is the specific heat of the solution and ΔT is the observed temperature change. Ap Chemistry Files 4 Designing A Hand Warmer The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch.
Calculate the heat released as the solid Designing A Hand Warmer Pre Lab Answers Heat approximately 125 mL of distilled water to 6070 C in a 250-mL beaker. Our People Custom Timber Frame Homes. 20 coupon applied at checkout Save 20 with.
Repeat steps 3 through 8 with the measurements 30ml and 25 grams for trial 2 and 20 ml and 4 grams for trial 3. Designing a Hand Warmer Lab Experiment OverviewThe purpose of this advanced inquiry lab is to design an effective hand warmer that is inexpensive nontoxic and safe for the environment. Designing an Effective Hand Warmer Designing a Hand Warmer Purpose.
Measure and record the temperature of the hot water. Designing a Hand Warmer AP Chemistry Big Idea 5 Investigation 12 An Advanced Inquiry Lab Introduction Put your chemistry skills to commercial use. The Chemistry Of Hand Warmers.
Based on the results of the lab hand warmers will benefit most with the use of LiCl since it raised the temperature of the solutions to about 19 degrees Celsius. 4252022 111418 PM. 𝐶cal 𝑞calor 𝑇mix𝑇𝑖nitial Equation 4.
Q hot -19000 J. 13- calculate calorimeter constant Part B and C. Designing an Effective Hand Warmer.
Designing A Hand Warmer Ap Lab Answers knowledge that people have search hundreds times for their favorite readings like this designing a hand warmer ap lab answers but end up in malicious downloads.

Designing A Hand Warmer Designing A Hand Warmer Purpose Of Experiment Research And Design An Studocu

Hhyuu Designing A Hand Warmer Lab 4 Page 1 Created With Publitas Com

Designing A Hand Warmer By Makayla Sabo

Ap Inquiry 12 Hand Warmer Challenge

Ap Chemistry Hand Warmer Lab Youtube

Hhyuu Designing A Hand Warmer Lab 4 Page 4 Created With Publitas Com
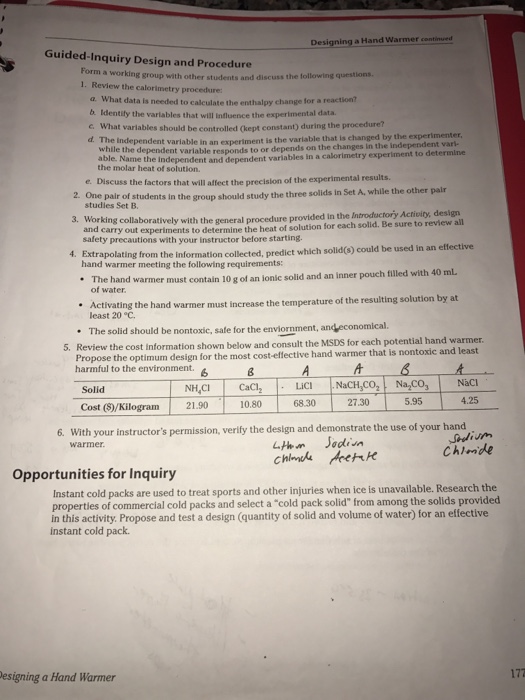
Solved Designing A Hand Warmer Continued Kn Chemistry Review Chegg Com

Solved Designing A Hand Warmer Continued Kn Chemistry Review Chegg Com
0 comments
Post a Comment